Scientists produce new treatment to block the development of breast cancer
By Benjamin Mateus
17 February 2018
The Medical College of Georgia last month said that a team of researchers had successfully used an inhibitor called HET0016 to block a chemical known as 20-HETE, which can promote the growth of breast cancer cells. The research was funded in part by the American Cancer Society and the National Institutes of Health.
Under normal conditions, 20-HETE metabolizes arachidonic acid, a polyunsaturated fatty acid that is present in cell membranes in the brain, muscles and liver. This reaction also helps regulate blood flow, blood pressure and inflammation and produces a host of different biologically active chemicals and metabolites necessary for proper cellular physiology and concerted signaling with other organ systems in our bodies.
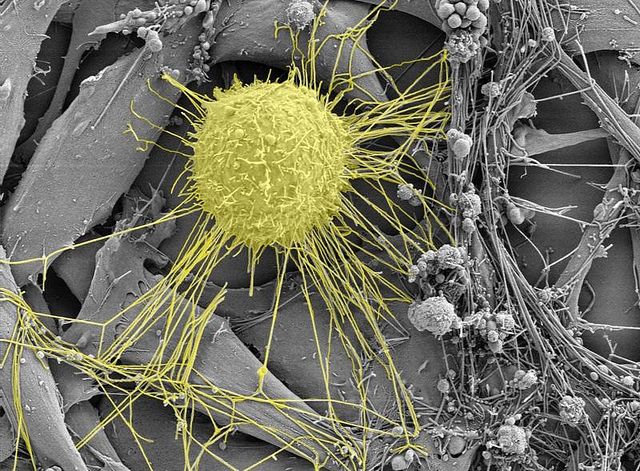 Breast cancer cell (yellow) as viewed through an electron microscope. Photo by Kristian Pfaller.
Breast cancer cell (yellow) as viewed through an electron microscope. Photo by Kristian Pfaller.
For instance, our neurological health is dependent on sufficient levels of arachidonic acid. It helps protect the brain from oxidative stress by activating proteins involved in growth and repair of neurons. During tissue injury, the acid is released from cell membranes, leading to important inflammatory mediators that help repair the damage. Conditions such as diabetes and obesity may lead to aberrations in these chemical processes.
The catch is that while 20-HETE and arachidonic acid stimulate various necessary intracellular pathways necessary for the proliferation, migration and survival of healthy cells, it seems that cancer also uses these mechanisms to protect itself.
Not all breast cancer is the same. There are four subtypes that demonstrate distinct morphology and have different clinical implications. They are designated by their molecular categories according to their steroid hormone receptor status, estrogen and progesterone, and human epidermal growth factor receptor 2. The differences account for different mortality rates and different required treatments.
The most common type of breast cancer, known as Luminal A, accounts for 30-70 percent of breast cancer and has the best prognosis, with high survival rates and low recurrence rates. The other three types—known as Luminal B, HER2-enriched and triple-negative breast cancer—all have lower survival rates, tending to grow in lymph nodes and exhibit more aggressive behaviors.
In general, the more aggressive types of breast cancer have a higher propensity for spreading to the bones, liver, lungs and brain. Patients with breast cancer in their bones have a median survival rate of between two and five years, while those diagnosed with brain cancer have the worst prognosis, with an approximate survival period of four to seven months. The current research is looking at ways to inhibit these more deadly cancers.
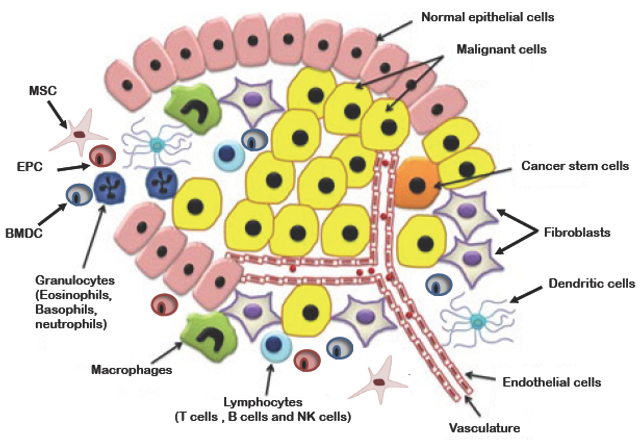 Primary Tumor Microenvironment: The tumor cells are surrounded by the normal epithelial cells including support cells such as Epithelial Progenitor cells (EPC), Bone Marrow Derived Cells (BMDC), and a host of white blood and mesenchymal cells that allow the cancer cells to thrive.
Primary Tumor Microenvironment: The tumor cells are surrounded by the normal epithelial cells including support cells such as Epithelial Progenitor cells (EPC), Bone Marrow Derived Cells (BMDC), and a host of white blood and mesenchymal cells that allow the cancer cells to thrive.
These particular tumor cells thrive in what are known as tumor microenvironments. Essentially the breast cancer cell is able to communicate with its surroundings and recruit tumor-associated cells, reprogram them to create new blood supplies for its growth and suppress immune cells from targeting it. This allows the breast cancer cell to reproduce and metastasize in a protected sanctuary.
By using HET0016, the authors were able to slow the growth of these tumors in animal models. They were then able to demonstrate decreased tumor volume, migration and invasion by the breast cancer cells which led to increased survival time. They also observed a synergistic reduction of the immune cell populations used by the cancer to protect itself, as well as a decrease in factors that promote the growth and development of new blood vessels. They were also able to demonstrate that HET0016 was able to decrease myelodysplastic cells, which are thought to diminish the immune system’s ability to attack the tumor cells.
The hypothesis that tumors need to grow new blood vessels in order to develop and expand was first proposed in 1971 by Judah Folkman. Since then cancer research has turned away from the concept of the tumor cells operating autonomously to meet their metabolic and nutritional needs and focused on understanding the interactions in the “tumor microenvironment,” which is comprised of normal support cells (called stromal), endothelial cells which line the interior of blood and lymphatic vessels, and immune cells of the individual which are reprogrammed to aid the tumor cells in surviving, growing and spreading.
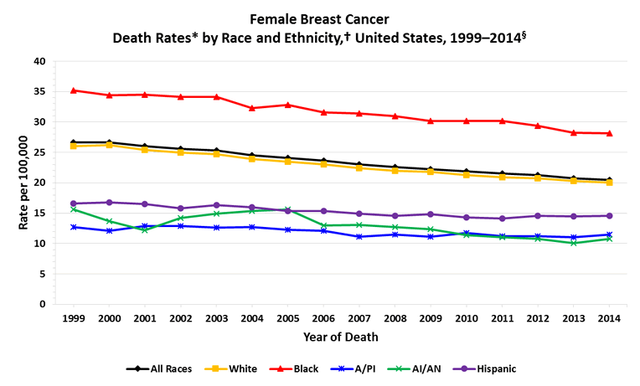 Cancer death rates from 1975-2020 for breast cancer from the Centers for Disease Control and Prevention, showing a decrease in rates as treatments have become more advanced and more available.
Cancer death rates from 1975-2020 for breast cancer from the Centers for Disease Control and Prevention, showing a decrease in rates as treatments have become more advanced and more available.
These findings have considerable social implications. Globally, breast cancer is the most common malignancy and the leading cause of death from cancer in women. Age, time to first menses, age at first live birth and age at menopause are known risk factors. Ten percent of breast cancer is attributable to hereditary factors. Modifiable risks such as demographics, lifestyle and environmental factors have not as yet been studied closely to determine the associations with breast cancer risk.
For instance, the incidence of breast cancer is higher in white women compared to black women, though black women more commonly present with regional or advanced disease than white women and have a higher breast cancer-specific mortality rate. Belying these figures are unmeasured factors such as access to health care or facility expertise that can detect these malignancies at much earlier stages. And yet in light of these and many other factors that deserve to be explored, there are very few studies exploring the role of 20-HETE inhibition in the treatment of malignancies.
Studies and research like this one not only offer insight into new treatment options for breast cancer but also highlight the complex interaction between external factors that promote inflammation caused by early life exposures, obesity, diabetes and ongoing social life stresses to include substance abuse and internal processes that promote the development of aggressive malignant cancers.
No comments:
Post a Comment